DOH says rapid tests still 'useful,' test maker explains how
MANILA, Philippines — A manufacturer of novel coronavirus disease 2019 (COVID-19) testing kits affirms the country's Department of Health (DOH) for clarifying that rapid antibody tests are still useful.
In a Monday briefing, DOH Undersecretary Maria Rosario Vergeire said rapid antibody tests should be used appropriately for them to be effective as rapid tests detect the presence of antibodies in the blood of people believed to have contracted COVID-19. This is after medical organizations have been sounding the alarm over the use of rapid antibody testing, saying results might create a false sense of security that may have contributed to the spread of COVID-19 in the Philippines.
Related: DOH: Rapid antibody tests not for screening COVID-19 cases but still useful
While the country's novel coronavirus disease 2019 (COVID-19) cases continue to rise and the possibility of another lockdown can cause panic and stress, one of the best things to do during these uncertain times is to be certain of one's health status by undergoing a reliable COVID-19 test.
Government and health authorities recommend getting tested to be sure of one's health, thus, securing the health of those who are around us.
In a bid to aid the country with the challenges of the pandemic and help detect the presence of immunoglobulin IgM and IgG antibodies to COVID-19 in a patient’s blood specimen, Jenka Global, a company that aims to showcase global-quality products, offer in vitro diagnostic rapid test to the Philippine market.
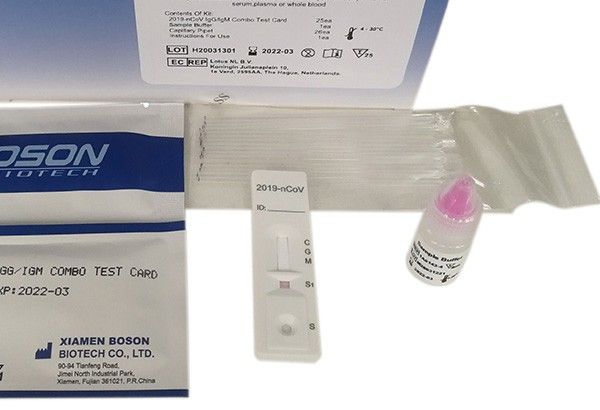
The Food and Drug Administration-approved Boson rapid test kit includes a combo test card8, sample buffer, capillary pipet and instructions for use. The kit must be kept at room temperature before testing. Only medical professionals, who follow the standard laboratory procedure and biosafety guidelines for handling and disposal of potentially infectious material should administer the test -- withdraw the blood specimen with the capillary pipet provided, add two drops of sample buffer to well, and then read the result at 15 minutes.
Boson rapid test meets the EN-ISO 13485 standards and has a sensitivity of 87.8 percent, the specificity of 99 percent, and accuracy of 96.8 percent.
Although the test is very accurate in detecting anti-COVID-19 antibody, a low incidence of false results can occur. Other clinically available tests are required if questionable results are obtained.
As with all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test but should only be made by the physician after all clinical and laboratory findings have been evaluated.
In the early stage of infection, it is suggested that patients should collect samples again after seven to 14 days and test at the same time as the last collected samples to confirm whether there is a serological positive transfer or significant increase in titer.
In the later stage of infection, IgM titer will decrease or even be negative, while IgG will continue to increase.
Manufactured by Xiamen Boson Biotech Co. Ltd., a specialist in the in-vitro diagnostic kits field, developing and manufacturing high-quality point-of-care and other immunoassay kits for the worldwide market, the test kit assures the public of its high accuracy.
According to Xiamen Boson Biotech's Quality Manager Liu Bin, "Our quality management system complies with ISO13485 and based on clinical data, our products have high accuracy. The sensitivity and specificity are the same for both asymptomatic and symptomatic COVID-19 patients. By using nucleocapsid and spike-RBD proteins, our test cards are highly sensitive to COVID-19 infections and are less likely to be affected by virus mutation. But all manufacturers inevitably encounter incidences of false positives and negatives. We can only ensure that each batch of products achieve the optimal quality through our strict control of raw materials, optimization of technical details, and strict quality control throughout our R&D and production processes."
However, according to the general guidelines for COVID-19, "a definitive clinical diagnosis should not be based on the result on the antibody tests alone. Rapid COVID-19 Testing should only be considered as part of a screening procedure. It should always be accompanied by a clinical and laboratory evaluation with consideration of a patient’s exposure, and symptoms, among others."



















