COVID-19 vaccine from China likely first to arrive in Philippines — vaccine czar
MANILA, Philippines (Updated 11:42 a.m.) — A Chinese-developed coronavirus vaccine may be the first to arrive in the Philippines, vaccine czar Carlito Galvez said Wednesday.
Galvez answered in the affirmative when asked in an interview on ANC’s “Headstart” if the first COVID-19 vaccine to be rolled out in the country would be from China.
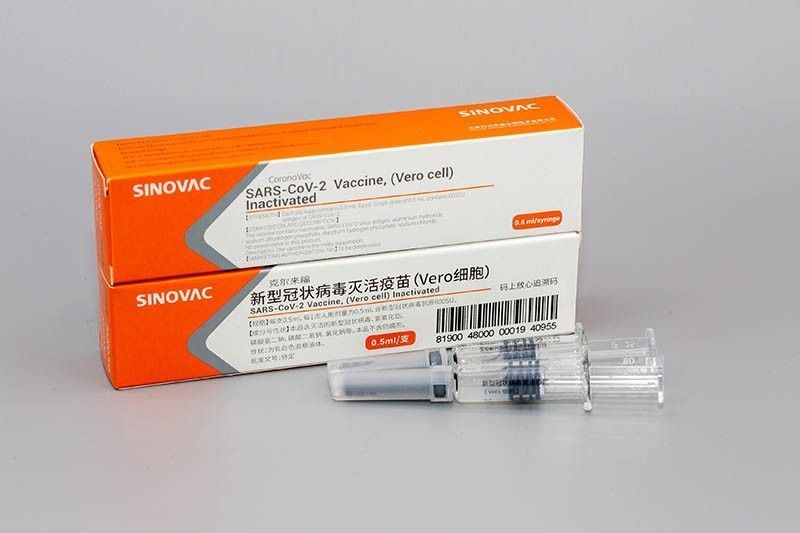
He said the coronavirus vaccine made by China’s Sinovac Biotech may be in the Philippines by March if a contract is signed within the month.
The vaccine developed by Russia’s Gamaleya Institute may also arrive in the country as early as the first quarter of 2021 but the vaccine czar said there may be “more confidence” in Sinovac.
“Most probably in the first quarter, either Gamaleya or Sinovac. But there is more confidence with Sinovac because Brazil and Indonesia had already gotten some of those,” Galvez said, partly in Filipino.
Indonesia has already received 1.2 million doses of the China-made vaccine over the weekend. Another 1.8 million doses are expected to be sent again in January.
Brazil's Sao Paulo state would begin large-scale immunization using Sinovac's vaccine next month.
Safety concerns
Sinovac Biotech, a private firm in China, has developed an inactivated coronavirus vaccine called “CoronaVac.”
Galvez also allayed fears over the safety of China-developed vaccines.
"I was briefed by the vaccine expert panel and based on their evaluation, Sinovac and the Chinese vaccines are very safe because it came from classical inactivated virus platform… They briefed me [that it is] the safest among the platforms and the classical that’s been used for years is the inactivated virus," he said.
According to the World Health Organization, inactivated vaccines are made from viruses or bacteria that have been killed through physical and chemical processes. These killed organisms cannot cause disease.
WHO also said inactivated vaccines are more stable than live attenuated vaccines. But these vaccines have “less strong” immune response compared to live vaccines.
Sinovac also applied to conduct Phase 3 clinical trials in the Philippines. The country’s Food and Drug Administration is evaluating Sinovac’s application, which was submitted in November, but documents are still lacking.
Aside from Sinovac, the Philippines is also in talks with Chinese firms Sinopharm and Cansino Biologics.
Vaccines from the West
Galvez said vaccines developed by other western pharmaceutical firms might arrive in the Philippines at a later date as majority of their supplies were already secured by wealthy nations and by their countries of origin.
United Kingdom became the first Western nation to begin vaccinating its citizens with Pfizer-BioNTech vaccines Tuesday.
The Philippine government is eyeing to inoculate around 24.7 million Filipinos in the first part of the vaccination program against COVID-19.
Some 1.76 million health workers are first in the list of priority beneficiaries for COVID-19 vaccination. Medical frontliners would be followed senior citizens, indigent Filipinos and uniformed personnel.
The Philippines, through the efforts of around 30 private companies, has so far secured at least 2.6 million doses of AstraZeneca vaccine, which has an average efficacy of 70%. The first doses of vaccine are expected to arrive in May or June 2020 after local regulatory approval. — with report from Agence France-Presse
- Latest
- Trending






























