DOH to seek emergency use authorization for Sinopharm COVID-19 vaccine
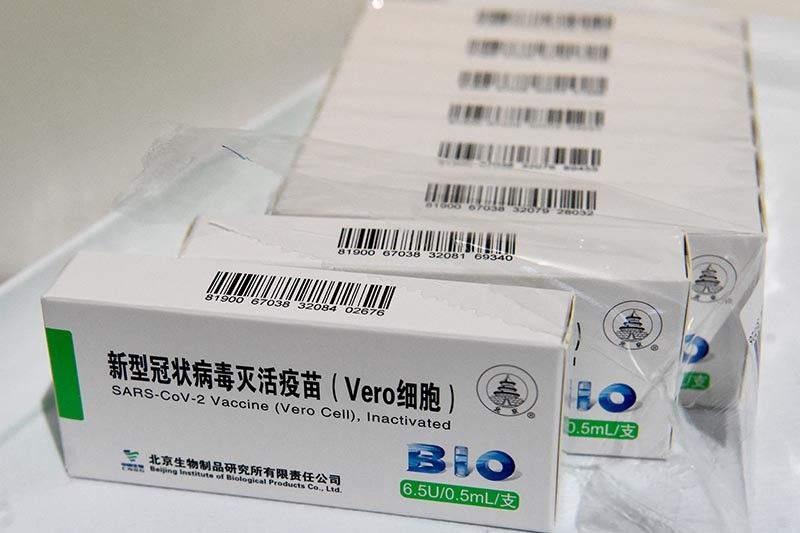
MANILA, Philippines (Updated 11:57 a.m.) — The Department of Health will file an application seeking an emergency use authorization for the COVID-19 vaccine developed by Chinese state-owned company Sinopharm, its chief said Monday.
In an interview with ABS-CBN’s Teleradyo, Health Secretary Francisco Duque III said the agency will submit an EUA application to the Food and Drug Administration after the World Health Organization approved the Sinopharm jab for emergency use.
In a text message to Philstar.com, FDA Director General Eric Domingo said “the government can apply for EUA.”
Executive Order 121, which allows the drug regulator to issue EUA for COVID-19 drugs and vaccines, states that an EUA application shall be submitted by "the industry or government agency concerned, such as the national procurer or the public health program implementer."
The FDA is an attached agency of the health department.
The FDA has issued EUAs to vaccines made by Pfizer and BioNTech, AstraZeneca, Sinovac, Gamaleya Research Institute, Johnson & Johnson, Bharat Biotech and Moderna.
The agency earlier said it has yet to start a formal review of EUA applications for Sinopharm as parties had yet to submit complete requirements. So far, the drug regulator had only granted a compassionate special permit to the Presidential Security Group.
Over the weekend, Duque said his department will seek an EUA for the Chinese jab so the Philippines will no longer have to return donated Sinopharm vaccines to Beijing.
President Rodrigo Duterte, who drew criticisms after he was given a Sinopharm shot even if it has yet to receive emergency use approval, said he will return China’s donated vaccine doses. A vial has been set aside for his second dose.
‘Not the first time’
In a separate briefing, Health Undersecretary Maria Rosario Vergeire said the department is in the process of collecting documents for the EUA application.
“This is not the first time that the DOH applied for EUA for new technologies like these vaccines. We have done this for COVAX Facility, for the donated Sinovac vaccines from China. This is something we do so we can facilitate the process of receiving vaccines,” Vergeire said in a mix of English and Filipino.
“We’re not going to represent any manufacturer for that matter. The reason we’re doing this is there’s a possibility that the transaction or negotiation for Sinopharm will be government to government,” she added.
The health official stressed that the EUA application will go through the usual regulatory process in the country.
The COVID-19 vaccine developed by Sinopharm became the first Chinese jab to receive the WHO’s green light. The United Nations health agency recommended the two-dose vaccine for adults 18 years and older.
The WHO also granted emergency use listing to the vaccines being made by Pfizer-BioNTech, Moderna, Johnson and Johnson, and AstraZeneca. An emergency use listing paves the way for nations to quickly approve and import a vaccine for distribution. — with report from Agence France-Presse
- Latest
- Trending