DOH to DFA: Mediate with Indian embassy for Remdesivir
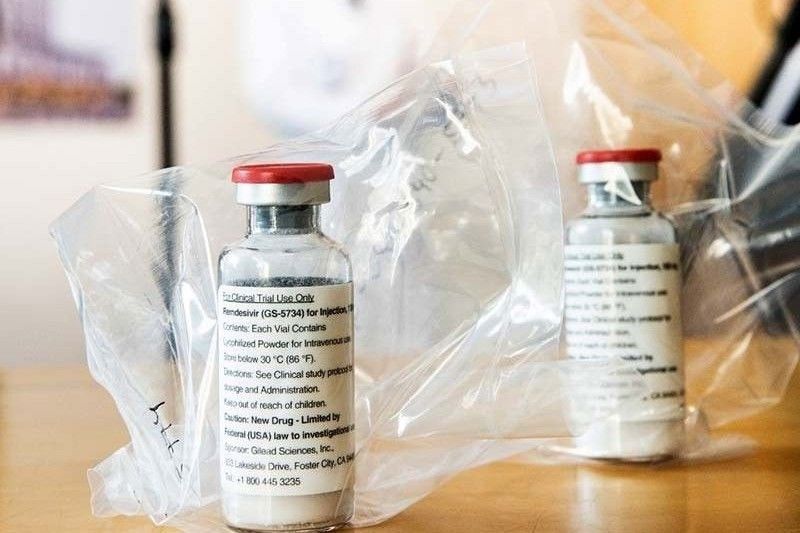
MANILA, Philippines — The Department of Health (DOH) has sought the help of the Department of Foreign Affairs (DFA) to convince the Indian embassy in Manila to release the Remdesivir drugs ordered by hospitals for their COVID-19 patients.
According to DOH Undersecretary Maria Rosario Vergeire, the delivery of an undisclosed amount of Remdesivir doses has been withheld when the Indian government banned the exportation of the antiviral drug.
“We asked the help of the DFA to write to the Indian embassy to relay our request to release the Remdesivir ordered by our COVID-19 hospitals,” she said yesterday at a press briefing.
Last April 11, India banned the exportation of Remdesivir as the country registered a record number of new daily COVID-19 cases.
A report of Bloomberg showed that seven Indian companies are producing Remdesivir under voluntary licensing agreement with Gilead Sciences Inc., an American biopharmacuetical firm.
Remdesivir is now an investigational drug for possible treatment for coronavirus disease.
Vergeire noted that Remdesivir does not have any registration yet with the Food and Drug Administration (FDA).
However, it is being used among COVID-19 patients in the country by virtue of the compassionate special permits (CSP) granted by FDA to requesting hospitals or doctors.
“The hospitals are the ones procuring through the suppliers because it’s CSP so the drug cannot be commercialized … It is available only in hospitals or doctors who applied for CSP,” she added.
Meanwhile, the initial 200,000 doses of Moderna COVID-19 vaccines to be delivered to the Philippines in June are allocated to the DFA and diplomatic missions in the country, Foreign Affairs Secretary Teodoro Locsin Jr. said yesterday.
“That’s DFA share; supposed to go to DFA staff and family and to Foreign Missions and all their staff,” Locsin said in a tweet. “I’ve told that to the diplomatic corps repeatedly and proudly announced it in international forums.”
Locsin was reacting to the statement of Philippine Ambassador to Washington Jose Manuel Romualdez on the commitment of Moderna to start its vaccine rollout in the Philippines by mid-June with the delivery of an initial 200,000 doses.
But the secretary learned that Moderna has yet to apply for emergency use authorization with the FDA.
The secretary announced in March that the entire diplomatic corps and their staff and personnel of DFA and their families will be inoculated with Moderna vaccine once the jabs from the American biotechnology company arrive in the country.
Locsin’s remark came after the announcement of the Israeli embassy in Manila that more than 30,000 Filipinos in Israel, including migrant workers, students, Philippine embassy staff and even those with expired working permits, have received free Pfizer COVID-19 vaccine as part of its successful vaccination program. – Pia Lee-Brago
- Latest
- Trending



























